Poly(ether)imides (PEI)
Properties
Poly(ether)imides (PI, PEI) are high performance engineering thermoplastics of amber to transparent color. They have outstanding thermal, mechanical, and chemical properties and are often the best choice for the most demanding applications where very high mechanical strength in combination with high temperature, corrosion, and wear resistance is required. For example, some grades have a continuous service temperatures of up to 700°F (371°C) and are suitable for short-term exposure up to 1000°F (538°C) with minimal thermal degradation and minimal loss of mechanical properties. PEIs and PIs resist most chemicals including hydrocarbons, alcohols and halogenated solvents and have excellent long-term creep resistance. In many cases, they can replace metals and other high performance materials in structural applications. The electrical properties are of excellent stability under variable temperature, humidity and frequency conditions.
Other important performance properties include:
- High tensile strength over a wide temperature range of about -270°C to + 300°C
- High compression strength and high pressure and creep resistance
- Excellent resistance to wear under high pressure and sliding speeds
- Excellent resistance to stress cracking
- Good cold temperature properties
- High glass transition temperature up to 400°C (amorphous resins)
- High melting temperature (semi-crystalline resins)
- Excellent long-term thermal-oxidative stability
- Inherently flame retardant
- Minimal thermal expansion
- High radiation resistance
- High purity and low outgassing in vacuum
- Good chemical resistance to acids, alcohols, fuels, oils, and halogenated solvents
- Excellent electrical insulation properties
- Low thermal conductivity
- Good processability (can be extruded, thermoformed, injection molded, etc.)
However, poly(ether)imides have also some limitations and shortcomings. For example, they are expensive and require high processing temperatures and they cannot be used above their glass transition temperature unless post annealed.
SYNTHESIS
Poly(ether)imides are typically infusible and insoluble due to their planar hetero-aromatic structure, and therefore, have to be processed via a solvent route. They are generally prepared by a two-step process from aromatic diamines and aromatic tetracarboxylic dianhydrides. The first steps of the condensation reaction is the addition of a dianhydride (pyromellitic dianhydride PMDA) to a diamine (4,4’-oxydianiline ODA) usually at ambient or low temperatures in a high boiling dipolar aprotic solvent, such as dimethyl sulfoxide (DMSO), N-methyl-2-pyrrolidone (NMP) or N,N-dimethylacetamide (DMAc). In some case, however, higher temperatures are required. The second step is a polycyclodehydration reaction of the poly(amic acid) which leads to the final polyimide with different molar mass depending on the composition.
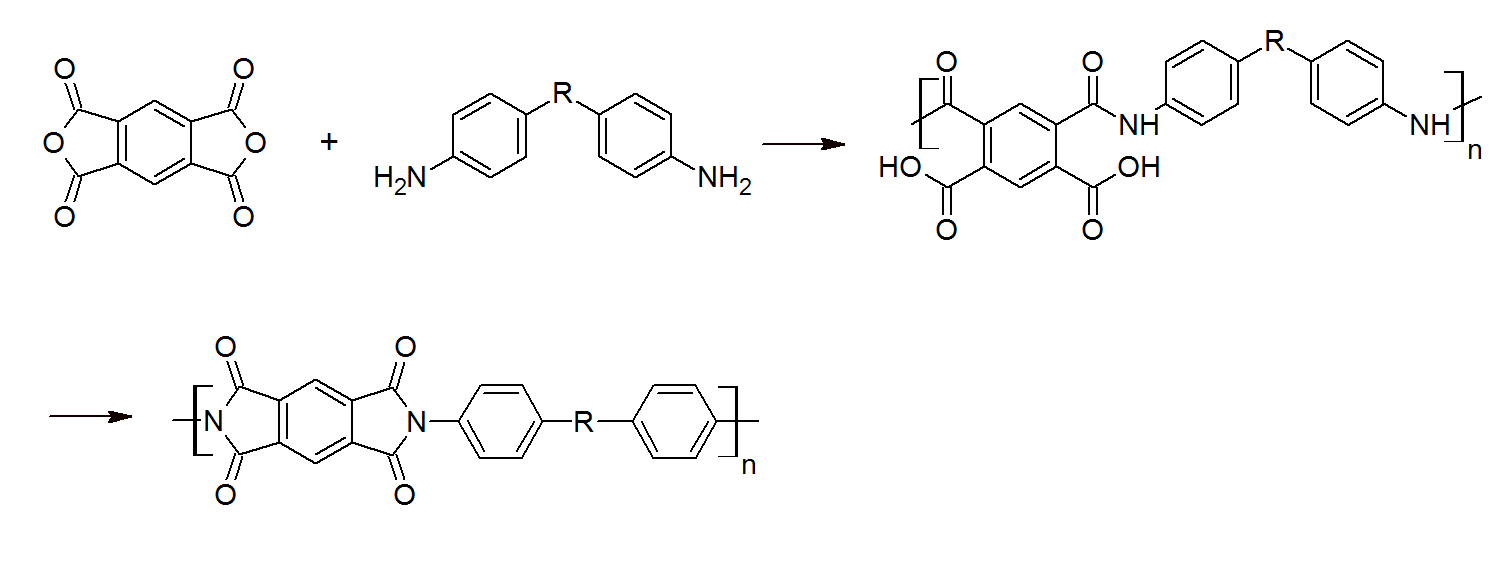
This process was utilized to produce the first polyimide of significant commercial importance - Kapton - which was synthesized from pyromellitic dianhydride (PMDA) and 4,4’-oxydianiline (ODA). In this case, R is an ether group. However, R can be any group.
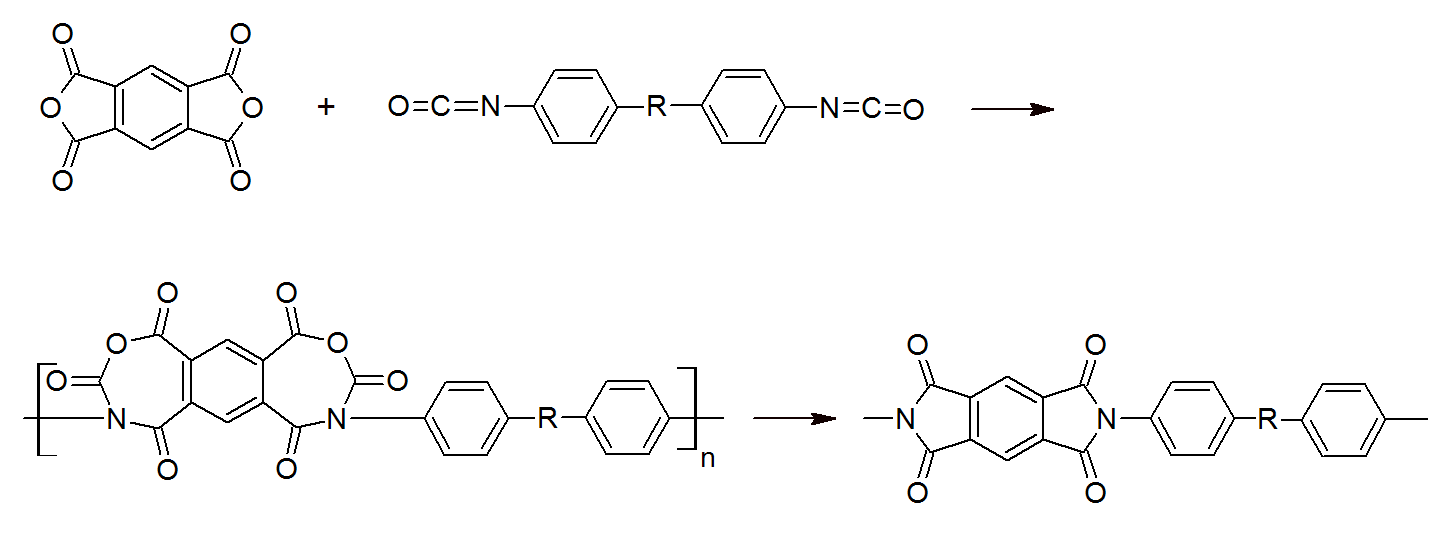
High molecular weight polyimides have also been prepared by the reaction of a diisocyanate with a dianhydride. This process is another two steps reaction. The first step is the addition of a dianhydride to a diisocyanate and the second step is a decarbonation reaction which leads to the final polyimide. This reaction is carried out in aprotic solvents.
A large variety of polyimides can be prepared from a large number of monomers. Even subtle variations in the structure of the dianhydride and diamine will have a significant effect on the properties of the final polyimide such as degree of crystallinity, glass transition temperature, and melting point. Chain stiffness and chain-chain interaction are undoubtedly the most important factors, which depend on the ratio and arrangement of flexible and rigid groups and the presence of bulky side groups.
The most common poly(ether)imides are synthesized from pyromellitic dianhydride or benzophenone tetracarboxylic dianhydride and 4,4-diamino diphenyl ether (oxy-dianiline) or methylenedianiline.
A major drawback of the methods above is the unavoidable presence of solvent and the formation of water or carbon dioxide during the condensation reaction. Both the condensation products and the solvent need to be fully removed prior post-processing of the resin to achieve high performance properties.
COMMERCIAL Polyetherimides
Poly(ether)imide (PEI) resins are manufactured by SABIC under the trade name ULTEM, as a result of acquiring the General Electric Plastics Division in 2007. PEI resins are also produced by Dupont and are sold under the trade name Kapton. The resins are available in transparent and opaque custom colors, as well as glass filled. The most common polyimides are synthesized from pyromellitic dianhydride and 4,4-diamino diphenyl ether or similar etherdiamines (Kapton type). However, some companines produce other poly(ether)imides for even higher heat, chemical, and/or elasticity requirements.
Applications
Poly(ether)imides are often an excellent choice for demanding applications in aerospace and transportation. They also find many applications in the electronic and integrated circuit industry because they meet the most demanding and strict materials specification. Some other important applications include probe housing, digital card printer frames, coil springs, and cable guards.
Because of their high price, polyimides and polyetherimides are usually only used when outstanding properties are required.
The typical service temperature range of polyetherimides is about -270°C to + 300°C.